Refractive Lens Exchange aka RLE|NLR + IOLs
- Edenvillage
- Offline
- Posts: 1
- Thanks: 0
This is exactly what I'm going through but I can't talk on the phone
_____________
admin I don’t understand the purpose of your post 👀
For help or advice, send email to: sasha@opticalexpressruinedmylife.co.uk
or
sasha@mybeautifuleyes.co.uk
_____________
admin I don’t understand the purpose of your post 👀
For help or advice, send email to: sasha@opticalexpressruinedmylife.co.uk
or
sasha@mybeautifuleyes.co.uk
Last Edit:08 Mar 2025 15:27
by Edenvillage
Please Log in or Create an account to join the conversation.
- admin
-
 Offline
Offline
- Posts: 1164
- Thanks: 153
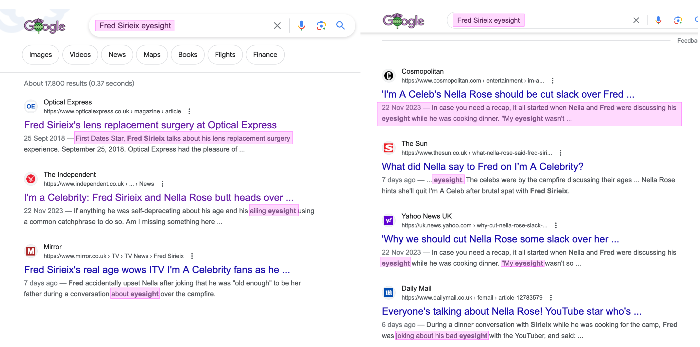
Googling 'Fred Sirieix eyesight', I was amazed to see the story has hit so many publications, all thanks to Nella Rose's serendipitous silliness 👀
More about I'M A CELEBRITY... GET ME OUT OF HERE! contestants to come 😉
Meanwhile, I'm surprised Optical Express haven't removed Fred's page yet, because his advertising is doing them more harm than good!
www.opticalexpress.co.uk/magazine/articl...t-surgery-experience
Please note that 'I'm A Celebrity...' related posts are now to be found in Press & Media Topic:
www.opticalexpressruinedmylife.co.uk/ind...tid=5&id=12560#21909
More about I'M A CELEBRITY... GET ME OUT OF HERE! contestants to come 😉
Meanwhile, I'm surprised Optical Express haven't removed Fred's page yet, because his advertising is doing them more harm than good!
www.opticalexpress.co.uk/magazine/articl...t-surgery-experience
Please note that 'I'm A Celebrity...' related posts are now to be found in Press & Media Topic:
www.opticalexpressruinedmylife.co.uk/ind...tid=5&id=12560#21909
Last Edit:01 Dec 2023 14:36
by admin
Please Log in or Create an account to join the conversation.
- admin
-
 Offline
Offline
- Posts: 1164
- Thanks: 153
Part 2 of 2 👀
I don’t watch reality TV shows, so had never heard of Nella Rose or Fred Sirieix until this clip was brought to my attention a few days ago.
www.youtube.com/shorts/qBC7x4V53oY
As seen in Pt 1, on 31 August 2018, tagging Optical Express , fred sirieix posted on his Facebook: ‘Off for lens replacement surgery with @OpticalExpress can't wait to see the results’
In the supposedly pre surgery part of his promo video, he says: ‘I want to be like the X-Men!’
Post surgery, allegedly 24 hrs later: ‘I’m very happy with the results, so, so far so good and I'm really looking forward to see what’s coming…’ (Was that a wink or squint at video end?)
It should be noted that Optical Express customers DO NOT get to see the surgeon the next day, even if needing emergency treatment, instead directed to NHS hospital!
'I was really pleased with the service I have received from the team at Optical Express – my surgeon was excellent, having performed over 80,000 procedures, so I was confident I was in safe hands.’
No Fred, you were definitely NOT in safe hands!
Dr Jan Venter has (and had) dozens of legal claims from his OE patients, and is also responsible for fitting #ICL lenses in Optimax Eye Surgery Specialists patients (before David Moulsdale poached him), negligently not informing them of the need for regular ECC checks, so that majority have been having their #ICLs removed, while others need urgent corneal grafts!
www.dailymail.co.uk/news/article-9934377...-damage-corneas.html
I have no doubt that Fred was incentivised to publicise OE with more than free surgery, a fact that neither he nor Optical Express have declared anywhere.*
In this ‘I'm A Celebrity’ clip, worrying about cutting his fingers, Fred says: ‘Well, I can’t see anything!’ (Oops, can X-Men see in the dark?)
And when Nella questions the fact that he had surgery, presumably remembering he signed a deal with OE, he doesn’t say much more, other than to tell her he was old (now 51), but I doubt anyone but a child would consider 46 old, which is, by the way, too young for lens exchange surgery.
Science stuff: Fred’s problem round the campfire at night is caused by the complex optics employed by multifocal intraocular lenses. They split the available light which causes loss of contrast perception and difficulty seeing in dim light.
The patient information booklet for the lenses I'm guessing he has (Tecnis multifocal ZKB00 or Tecnis Symfony EDOF) actually warns of this, for example, that patients may have difficulty perceiving hazards on the road when driving at night.
Both lenses also cause dysphotopsia (arcs/streaks/glare/halos/starbursts around light sources).
And if he has the EDOF lenses, his near vision will be sh*t!
With Fred in the jungle and unable to tell me himself, I will be contacting his Encanta Talent Management agent to confirm or deny if payment was received for this promo.
I don’t watch reality TV shows, so had never heard of Nella Rose or Fred Sirieix until this clip was brought to my attention a few days ago.
www.youtube.com/shorts/qBC7x4V53oY
As seen in Pt 1, on 31 August 2018, tagging Optical Express , fred sirieix posted on his Facebook: ‘Off for lens replacement surgery with @OpticalExpress can't wait to see the results’
In the supposedly pre surgery part of his promo video, he says: ‘I want to be like the X-Men!’
Post surgery, allegedly 24 hrs later: ‘I’m very happy with the results, so, so far so good and I'm really looking forward to see what’s coming…’ (Was that a wink or squint at video end?)
It should be noted that Optical Express customers DO NOT get to see the surgeon the next day, even if needing emergency treatment, instead directed to NHS hospital!
'I was really pleased with the service I have received from the team at Optical Express – my surgeon was excellent, having performed over 80,000 procedures, so I was confident I was in safe hands.’
No Fred, you were definitely NOT in safe hands!
Dr Jan Venter has (and had) dozens of legal claims from his OE patients, and is also responsible for fitting #ICL lenses in Optimax Eye Surgery Specialists patients (before David Moulsdale poached him), negligently not informing them of the need for regular ECC checks, so that majority have been having their #ICLs removed, while others need urgent corneal grafts!
www.dailymail.co.uk/news/article-9934377...-damage-corneas.html
I have no doubt that Fred was incentivised to publicise OE with more than free surgery, a fact that neither he nor Optical Express have declared anywhere.*
In this ‘I'm A Celebrity’ clip, worrying about cutting his fingers, Fred says: ‘Well, I can’t see anything!’ (Oops, can X-Men see in the dark?)
And when Nella questions the fact that he had surgery, presumably remembering he signed a deal with OE, he doesn’t say much more, other than to tell her he was old (now 51), but I doubt anyone but a child would consider 46 old, which is, by the way, too young for lens exchange surgery.
Science stuff: Fred’s problem round the campfire at night is caused by the complex optics employed by multifocal intraocular lenses. They split the available light which causes loss of contrast perception and difficulty seeing in dim light.
The patient information booklet for the lenses I'm guessing he has (Tecnis multifocal ZKB00 or Tecnis Symfony EDOF) actually warns of this, for example, that patients may have difficulty perceiving hazards on the road when driving at night.
Both lenses also cause dysphotopsia (arcs/streaks/glare/halos/starbursts around light sources).
And if he has the EDOF lenses, his near vision will be sh*t!
With Fred in the jungle and unable to tell me himself, I will be contacting his Encanta Talent Management agent to confirm or deny if payment was received for this promo.
Last Edit:26 Nov 2023 20:05
by admin
Please Log in or Create an account to join the conversation.
- admin
-
 Offline
Offline
- Posts: 1164
- Thanks: 153
'First Dates Star, Fred Sirieix talks about his lens replacement surgery experience
September 25, 2018
'Optical Express had the pleasure of welcoming First Dates Star and TV personality Fred Sirieix to the London Westfield clinic for lens replacement surgery this August.
'"Since undergoing the procedure. Gradually, my eyes adjusted and my sight has improved day by day. My vision is a lot brighter, I can see colours I have never seen before and everything in so much detail – like the X-Men!"’
www.opticalexpress.co.uk/magazine/articl...t-surgery-experience ?
NB: For those unfamilar with his image, Fred's surgeon was Dr Jan Venter, who I note has changed his hair colour from carrot to deep mahogany since I last saw him in person, at his Irish Medical Council Fitness to Practise hearing in Dublin in 2017: www.opticalexpressruinedmylife.co.uk/ind...opic&catid=5&id=7815
As Fred is currently in the Australian 'jungle', I can't yet ask if he was paid for his OE promo!
September 25, 2018
'Optical Express had the pleasure of welcoming First Dates Star and TV personality Fred Sirieix to the London Westfield clinic for lens replacement surgery this August.
'"Since undergoing the procedure. Gradually, my eyes adjusted and my sight has improved day by day. My vision is a lot brighter, I can see colours I have never seen before and everything in so much detail – like the X-Men!"’
www.opticalexpress.co.uk/magazine/articl...t-surgery-experience ?
NB: For those unfamilar with his image, Fred's surgeon was Dr Jan Venter, who I note has changed his hair colour from carrot to deep mahogany since I last saw him in person, at his Irish Medical Council Fitness to Practise hearing in Dublin in 2017: www.opticalexpressruinedmylife.co.uk/ind...opic&catid=5&id=7815
As Fred is currently in the Australian 'jungle', I can't yet ask if he was paid for his OE promo!
Last Edit:25 Nov 2023 16:15
by admin
Please Log in or Create an account to join the conversation.
- Barbara
I could have written that myself 😢
Last Edit:08 Mar 2025 15:27
by Barbara
Please Log in or Create an account to join the conversation.
- Dale
- Offline
- Posts: 1
- Thanks: 0
Hello, i had my surgery around 14 years ago, and all has been fine until this year when my right eye has started to look steamed up and not very clear so l booked an oppointment at OE medowhall i was told something had started to grow on my lense and that i will have to be referred to the NHS to sort this out???????
by Dale
Please Log in or Create an account to join the conversation.
- n.khan
- Thanks: 0
Hi I visited OE on Monday luckily not paid anything just quoted 8500 for NLR surgery as I need cataract and astigmatism corrected. Omg so shocked to see this site!. I told them I’d like to think about it,
My question is RLE a bad thing. Should I just get monofocal on nhs. Wanted to do this to get rid of glasses. I have booked a consultation with optegra as well and moorfields OE did deliver a hard pitch, I signed up online and Sunday 9am started getting calls upon calls. Thought let me go and check out, thank God for this site. So do the people on this forum who have been affected advise against this treatment I have prematurely developed cataract at 48 and need it removed thought might explore removing need to wear glasses as I am very short sighted too.
I really I could get done but looks like so many risks maybe I’ll just have done on nhs and have the standard procedure. Is there anyone who’s had a successful outcome???
OE called to ask about what I want to do. I told them straight up I’ve googled a website called OE RUINED MY LIFE. They said they are aware and also said the procedures were done by another company that OE bought and now OE are being blamed. But I told them straight up the website has made me feel scared.
Thanks again.
Can you call me if I email?
Naheed khan
———————————
admin: All private providers have damaged patients, and only give 12 months free aftercare!
If you actually have cataracts, I strongly recommend that you stick with the NHS.
As for OE’s damaged patients being inherited from another company they bought - 🤣
For more info please send your phone number
sasha@opticalexpressruinedmylife.co.uk
My question is RLE a bad thing. Should I just get monofocal on nhs. Wanted to do this to get rid of glasses. I have booked a consultation with optegra as well and moorfields OE did deliver a hard pitch, I signed up online and Sunday 9am started getting calls upon calls. Thought let me go and check out, thank God for this site. So do the people on this forum who have been affected advise against this treatment I have prematurely developed cataract at 48 and need it removed thought might explore removing need to wear glasses as I am very short sighted too.
I really I could get done but looks like so many risks maybe I’ll just have done on nhs and have the standard procedure. Is there anyone who’s had a successful outcome???
OE called to ask about what I want to do. I told them straight up I’ve googled a website called OE RUINED MY LIFE. They said they are aware and also said the procedures were done by another company that OE bought and now OE are being blamed. But I told them straight up the website has made me feel scared.
Thanks again.
Can you call me if I email?
Naheed khan
———————————
admin: All private providers have damaged patients, and only give 12 months free aftercare!
If you actually have cataracts, I strongly recommend that you stick with the NHS.
As for OE’s damaged patients being inherited from another company they bought - 🤣
For more info please send your phone number
sasha@opticalexpressruinedmylife.co.uk
Last Edit:26 Sep 2023 15:08
by n.khan
Please Log in or Create an account to join the conversation.
- Maria Colman
Replied by Maria Colman on topic Lens replacement WORST decision of my life!
Posted 23 Apr 2023 08:23 #8
I have had exactly the same problems. My near sight is worse than before the operation. I have halos and starbursts at night time so can't do night time driving. It is affecting my job as I do close up work. I also have reaction to light. They have told me there is nothing more they can do other than replacing my lens with mono lens!! I wish I had never had this done now. It seems to be much more common than they actually tell you.
—————————————
admin: Hi Maria, if you haven’t yet done so, before risking your eyes to OE again, please contact me with your phone number
sasha@opticalexpressruinedmylife.co.uk
—————————————
admin: Hi Maria, if you haven’t yet done so, before risking your eyes to OE again, please contact me with your phone number
sasha@opticalexpressruinedmylife.co.uk
Last Edit:23 Apr 2023 16:14
by Maria Colman
Please Log in or Create an account to join the conversation.
- RobNow
-
 Offline
Offline
- Posts: 1
- Thanks: 0
I regret that I found this site after my surgery in Optical Express but I hope it's not too late to save my eyesight.
Sorry for my writing style and poor English grammar.
My name is Robert I am 50 years old and I live in Warwick.
I decided to have this surgery because my presbyopia was progressing very fast,I had problem with short distance vision like reading, see something close to my eye,which made it very difficult for me to do my job.But my long distance sight was really really good.
On October 26th I had NLR surgery [RLE] at Birmingham Optical Express.
The surgery was a complete failure and left my vision worse than it was prior to the surgery. I suffered from double/ghosting vision daytime and halos and starbursts at night time.
During each of my visits after NLR, I kept trying to explain that my eyesight wasn't getting better, I was convinced every time that my eyesight would improve soon, first they said it would take a week, then it would take a month, then after three months.
Finally after 4 months they say: everything be alright we do for you laser surgery correction.I recived temporary contact lens (I waited for these lens for a whole month)
Unfortunately, after putting them on, it turned out that my vision defect is even more serious.I told them that I still see double etc. At long distance I can't read signs and inscriptions,I can't recognize people.Because I can see much better in the short distance I feel and see the same when I put on my reading glasses before the surgery.
So I was told that at the moment they don't know how to help me and they have to consult my surgeon on what to do next. That was the first time I felt helplessness and fear,that it could all end badly, that something was wrong with my eyes, that the surgery had not been done as it should have been.
Today after another three weeks I got a call. I thought they would give me an appointment with the surgeon of the same clinic where I had the surgery. Unfortunately,they told me that I have an appointment on April 14th and I will receive temporary glasses. I didn't expect it at all - Glasses?! what glasses?! I saved for 5 years to keep wearing glasses!?
I didn't know what to say I started to stutter and said:Yes,I will come. Of course... I will come.....Thank you.....
I have been taking a high dose of antidepressant for 10 years so I can say that I have a lot of experience how to fight with my stress and anxiety. I am very worried about that. Can I ask for your advice on how to talk to them and what to do please? I'll wait patiently I realize I'm not the only one asking for your help.I'll be very grateful to you for any help.
_______________________
admin: Hi Robert, please send me your phone number asap
sasha@opticalexpressruinedmylife.co.uk
Sorry for my writing style and poor English grammar.
My name is Robert I am 50 years old and I live in Warwick.
I decided to have this surgery because my presbyopia was progressing very fast,I had problem with short distance vision like reading, see something close to my eye,which made it very difficult for me to do my job.But my long distance sight was really really good.
On October 26th I had NLR surgery [RLE] at Birmingham Optical Express.
The surgery was a complete failure and left my vision worse than it was prior to the surgery. I suffered from double/ghosting vision daytime and halos and starbursts at night time.
During each of my visits after NLR, I kept trying to explain that my eyesight wasn't getting better, I was convinced every time that my eyesight would improve soon, first they said it would take a week, then it would take a month, then after three months.
Finally after 4 months they say: everything be alright we do for you laser surgery correction.I recived temporary contact lens (I waited for these lens for a whole month)
Unfortunately, after putting them on, it turned out that my vision defect is even more serious.I told them that I still see double etc. At long distance I can't read signs and inscriptions,I can't recognize people.Because I can see much better in the short distance I feel and see the same when I put on my reading glasses before the surgery.
So I was told that at the moment they don't know how to help me and they have to consult my surgeon on what to do next. That was the first time I felt helplessness and fear,that it could all end badly, that something was wrong with my eyes, that the surgery had not been done as it should have been.
Today after another three weeks I got a call. I thought they would give me an appointment with the surgeon of the same clinic where I had the surgery. Unfortunately,they told me that I have an appointment on April 14th and I will receive temporary glasses. I didn't expect it at all - Glasses?! what glasses?! I saved for 5 years to keep wearing glasses!?
I didn't know what to say I started to stutter and said:Yes,I will come. Of course... I will come.....Thank you.....
I have been taking a high dose of antidepressant for 10 years so I can say that I have a lot of experience how to fight with my stress and anxiety. I am very worried about that. Can I ask for your advice on how to talk to them and what to do please? I'll wait patiently I realize I'm not the only one asking for your help.I'll be very grateful to you for any help.
_______________________
admin: Hi Robert, please send me your phone number asap
sasha@opticalexpressruinedmylife.co.uk
Last Edit:05 May 2023 15:55
by RobNow
Please Log in or Create an account to join the conversation.
- bill3066
- Offline
- Posts: 1
- Thanks: 0
Hi Im hoping that someone can advise me as to how I can get my eyes looked at and my eyesight improved after OE performed an RLE in 2014 after telling me on my initial consolation that I have the start of cataracts. I have had YAG treatment pin both eyes once in 2016 and then in 2021 both times by NHS.
I have been back to OE on two occasions and they have admitted that the lenses they have implanted have both opacified and both need changing but after presenting myself to Westfields for the procedure was advised by the Optometrist that one eye would not be improved due to ERM, was also told that any referral to hospital would not happen because the vision in one eye was acceptable despite both lenses are opacified.
I went to my GP who then sent me to local NHS hospital and the Senior Optometrist there said that this was not the case and more likely that the bad vision was due to the lens moving around,
I have really lost faith in these people and would like to get someone who is competant.to rectify the error.
This is an abridged version of the whole story
Bill
___________________
admin: Please send your phone number to sasha@opticalexpressruinedmylife.co.uk
Last Edit:17 Nov 2022 08:33
by bill3066
Please Log in or Create an account to join the conversation.
Moderators: admin, Sasha