Faulty Oculentis Lenses
- Sandra Batchelor
I had lense replacement at optical express 2012. My eye sight is getting worse especially in one eye. I was advised I would need laser (YAG) as there were a fault with the batch of lenses I visited the clinic today and got told the yag procedure does not work. I would need the lense replaced but there is a long waiting list.
Please advise me as I am so worried
Thanks
________________________
admin: Please send email 👀
sasha@opticalexpressruinedmylife.co.uk
Please advise me as I am so worried
Thanks
________________________
admin: Please send email 👀
sasha@opticalexpressruinedmylife.co.uk
Last Edit:18 Feb 2024 16:28
by Sandra Batchelor
Please Log in or Create an account to join the conversation.
- PeterC
- Offline
- Posts: 1
- Thanks: 0
My Wife was fitted with the occulentis lenses in 2015, she has had the right eye lenses explanted in October 24, by Optical Express as the NHS would not do it and after weeks of back and forth with Optical Express, they eventual agreed to change the lense, We are now looking into making a claim against Optical Express but did not want to go with the miriad of ambulance chasers on the internet.
Any advice would be greatly received
_______________
admin: Please email me for advice 👀
sasha@opticalexpressruinedmylife.co.uk
Any advice would be greatly received
_______________
admin: Please email me for advice 👀
sasha@opticalexpressruinedmylife.co.uk
Last Edit:05 Jan 2024 05:21
by PeterC
Please Log in or Create an account to join the conversation.
- Heron26
- Offline
- Posts: 1
- Thanks: 0
Hello
I’m hoping someone here can offer me advice please.
I had lens replacement surgery at Optical Express in 2014 and until 2020; no problems at all.
Then started to have very blurred vision so when to Moorfields eye hospital when they have seen me every 6 months to monitor my vision.
I’ve now been told that the lenses used in both eyes are from a faulty Oculentis batch and only option for me is to have them replaced which is quite a risky operation. Has anyone had this done and how did it go and also is the a way I can claim back my original money and also for the money it’s going to cost me to have them placed privately at Moorfields which is around £10,000.
Thanks for any advice
Jan
____________________-
admin: Please email me for advice
sasha@opticalexpressruinedmylife.co.uk
I’m hoping someone here can offer me advice please.
I had lens replacement surgery at Optical Express in 2014 and until 2020; no problems at all.
Then started to have very blurred vision so when to Moorfields eye hospital when they have seen me every 6 months to monitor my vision.
I’ve now been told that the lenses used in both eyes are from a faulty Oculentis batch and only option for me is to have them replaced which is quite a risky operation. Has anyone had this done and how did it go and also is the a way I can claim back my original money and also for the money it’s going to cost me to have them placed privately at Moorfields which is around £10,000.
Thanks for any advice
Jan
____________________-
admin: Please email me for advice
sasha@opticalexpressruinedmylife.co.uk
Last Edit:21 Jun 2023 14:21
by Heron26
Please Log in or Create an account to join the conversation.
- Anonymous Friend
Incase you didn't know, Teleon has sold to the Chinese company Gaush, their Chinese distributor. Gaush is now going public in HK and had to post the details of the purchase..
www1.hkexnews.hk/app/sehk/2021/104028/do.../sehk21112800135.pdf
Looks like deal around 171 Million Euro...
I hope those that are expecting get what they are owed..
www1.hkexnews.hk/app/sehk/2021/104028/do.../sehk21112800135.pdf
Looks like deal around 171 Million Euro...
I hope those that are expecting get what they are owed..
Attachment not found
Attachment not found
by Anonymous Friend
Please Log in or Create an account to join the conversation.
- admin
-
 Offline
Topic Author
Offline
Topic Author
- Posts: 1164
- Thanks: 153
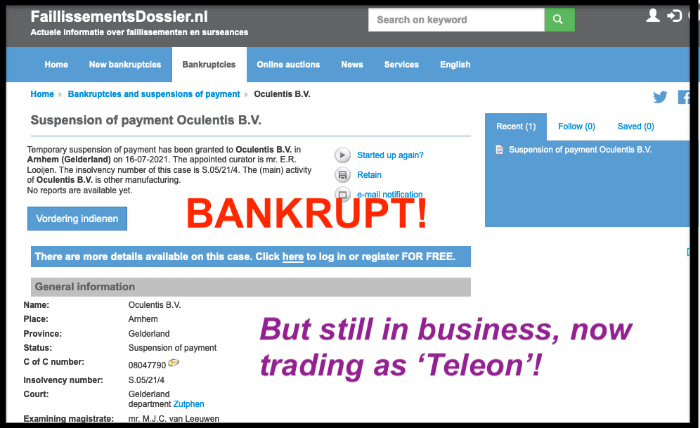
Posted 30 July 2021: 'As I predicted when they started trading as Teleon in 2020, Oculentis has declared bankruptcy...'
But whilst lawyers representing so many clients fitted with Oculentis faulty lenses battle on with legal claims, and victims needing explants burden nhs.uk, the Oculentis management team are laughing!
Because, before putting Oculentis into administration last year,
CEO Ben Wanders set up Teleon-Surgical, enabling his company to continue trading uninterrupted and unecumbered by debt or responsibility.
Although most of the Oculentis team are listed on Teleon-Surgical website, I note that Mark Lansu (still wearing specs!) has now replaced Ben Wanders as CEO.
www.teleon-surgical.com/en/international/about-us/team/
Yet another unscrupulous and unethical company, caring only about profits, without regard for so many customers whose lives they've contributed to ruining!
NB: I have been advised by one lawyer representing a number of Optical Express and Optegra Eye Hospital customers fitted with faulty lenses that claims are continuing.
If you need advice re the above, and not already in litigation, please contact: sasha@mybeautifuleyes.co.uk :kiss:
But whilst lawyers representing so many clients fitted with Oculentis faulty lenses battle on with legal claims, and victims needing explants burden nhs.uk, the Oculentis management team are laughing!
Because, before putting Oculentis into administration last year,
CEO Ben Wanders set up Teleon-Surgical, enabling his company to continue trading uninterrupted and unecumbered by debt or responsibility.
Although most of the Oculentis team are listed on Teleon-Surgical website, I note that Mark Lansu (still wearing specs!) has now replaced Ben Wanders as CEO.
www.teleon-surgical.com/en/international/about-us/team/
Yet another unscrupulous and unethical company, caring only about profits, without regard for so many customers whose lives they've contributed to ruining!
NB: I have been advised by one lawyer representing a number of Optical Express and Optegra Eye Hospital customers fitted with faulty lenses that claims are continuing.
If you need advice re the above, and not already in litigation, please contact: sasha@mybeautifuleyes.co.uk :kiss:
Last Edit:19 Sep 2021 18:56
by admin
Please Log in or Create an account to join the conversation.
- admin
-
 Offline
Topic Author
Offline
Topic Author
- Posts: 1164
- Thanks: 153
As I predicted in my 27 August 2020 post, when they started trading as Teleon in 2020, Oculentis has declared bankruptcy 
www.faillissementsdossier.nl/en/suspensi...7/oculentis-b-v.aspx
Solicitors representing so many clients fitted with faulty Oculentis lenses have yet to find out where that leaves current legal claims against the company.
But more importantly, what happens to the 'Patient Pathway' for those who still need the faulty lenses explanting?
Optical Express and Optegra Eye Hospital fitted many of these lenses, but I know they won't offer to explant for free if Oculentis are no longer funding.
Another burden for the NHS!
Shame on all involved, including distributors Topcon.
NB: This is just one VERY good reason not to risk unregulated and unnecessary lens exchange or cataract surgery with private companies, because they might not be around to provide aftercare if there're problems at a later date!
For example, Optimax has already declared insolvency and entered a Company Voluntary Arrangement (CVA), which means they could be forced into administration (bankruptcy) very soon if they're unable to pay their creditors - of which it seems I'm one, according to owner Russell Ambrose!
www.faillissementsdossier.nl/en/suspensi...7/oculentis-b-v.aspx
Solicitors representing so many clients fitted with faulty Oculentis lenses have yet to find out where that leaves current legal claims against the company.
But more importantly, what happens to the 'Patient Pathway' for those who still need the faulty lenses explanting?
Optical Express and Optegra Eye Hospital fitted many of these lenses, but I know they won't offer to explant for free if Oculentis are no longer funding.
Another burden for the NHS!
Shame on all involved, including distributors Topcon.
NB: This is just one VERY good reason not to risk unregulated and unnecessary lens exchange or cataract surgery with private companies, because they might not be around to provide aftercare if there're problems at a later date!
For example, Optimax has already declared insolvency and entered a Company Voluntary Arrangement (CVA), which means they could be forced into administration (bankruptcy) very soon if they're unable to pay their creditors - of which it seems I'm one, according to owner Russell Ambrose!
Last Edit:30 Jul 2021 19:22
by admin
Please Log in or Create an account to join the conversation.
- Anonymous
- admin
-
 Offline
Topic Author
Offline
Topic Author
- Posts: 1164
- Thanks: 153
(3 of 3)
I understand that Oculentis is now trading as Teleon-Surgical
The very negative press (worldwide) that Oculentis has accumulated over the past few years must be very worrying for Ben Wanders, so entirely understandable that he would want to disentangle his products from this (deserved) reputation.
Google 'Oculentis’ and the first TEN pages are filled with countless ads from law firms jostling for clients* fitted with faulty Lentis lenses, and press reports, that also mention the problematic MPlusX lenses - and of course links to this website and OERML Facebook page.
*Warning re two ambulance chasing law firms to avoid posted 28 July & 18 August - contact me for details!
And whilst I don't profess to be an expert in corporate structure, I suspect that’s why Oculentis set up a second company!
Teleon appears to sell the same products, owned by the same CEO, has the same MD, and shares many of the same 'Team’ members!
www.teleon-surgical.com/en/national/service/legal-notice/
www.teleon-surgical.com/en/international/about-us/team/Teleon
The Teleon website was published earlier this year, its design quite amateurish and childlike, but I suspect this may be temporary, because click on the ‘PRODUCTS’ tab and the ‘LENTIS | FEMTIS' drop down link currently connects to the Oculentis site.
As I said, I'm not an expert in corporate structure, but I wonder if Oculentis plan to go into administration, or take other steps to swerve responsibility for further expected legal claims?
AND - for whatever reason - should the company no longer exist, where would that leave damaged UK patients needing to access the Oculentis 'Patient Pathway’?
Answer: NHS!
And whilst his recent photo suggests that Oculentis |Teleon MD Mark Lansu has the same (lack of) faith in their lenses as does TopconGB MD Andrew Yorke, the latter has not responding to my perfectly polte and reasonable email last week...
'From: SashaMBEF <sasha@mybeautifuleyes.co.uk>
Subject: Oculentis Patient Pathway (OPP)
Date: 19 August 2020 at 16:27:10 BST
To: Andrew.Yorke@topcon.co.uk
Cc: mark.lansu@oculentis.com, ben.Wanders@oculentis.com
Hi Andrew
Whilst you and your colleagues have refused to respond to my questions since 2015 (selection below), I am aware that both Topcon and Oculentis regularly monitor my social media sites, and have read my recent posts.
Regardless of your hostility toward me, it is a fact that, as patient advocate with My Beautiful Eyes Foundation, I am the only person in the UK providing help and advice to the many thousands of people damaged by refractive eye surgery, thanks to the government’s refusal to address this massive scandal, with corruption at every level (globally).
Within the last week MBEF has been contacted by three more people who recently attended Optical Express stores complaining of visual problems, all shocked to be told that they have the faulty Lentis IOLs - and I have no doubt there are more yet to come!
However, none of these people want to have the ‘free’ treatment offered by OE (paid by Oculentis) and have asked for my advice.
It is possible that litigation could be avoided in at least one of these cases, the person only concerned with having the problematic calcified lenses removed asap, by an experienced surgeon of their choice.
Unfortunately, if they are unable to access the OPP directly, there will be no alternative but to litigate.
With that in mind, I am asking you the same question that Ben Wanders has refused to answer.
Can you please provide me with details of the Oculentis 'Patient Pathway’, and how my clients can access it if not currently in litigation?
I look forward to your earliest response.
Best wishes ?
Sasha Rodoy | My Beautiful Eyes Foundation
Patient Advocate & Campaign Manager'
I understand that Oculentis is now trading as Teleon-Surgical
The very negative press (worldwide) that Oculentis has accumulated over the past few years must be very worrying for Ben Wanders, so entirely understandable that he would want to disentangle his products from this (deserved) reputation.
Google 'Oculentis’ and the first TEN pages are filled with countless ads from law firms jostling for clients* fitted with faulty Lentis lenses, and press reports, that also mention the problematic MPlusX lenses - and of course links to this website and OERML Facebook page.
*Warning re two ambulance chasing law firms to avoid posted 28 July & 18 August - contact me for details!
And whilst I don't profess to be an expert in corporate structure, I suspect that’s why Oculentis set up a second company!
Teleon appears to sell the same products, owned by the same CEO, has the same MD, and shares many of the same 'Team’ members!
www.teleon-surgical.com/en/national/service/legal-notice/
www.teleon-surgical.com/en/international/about-us/team/Teleon
The Teleon website was published earlier this year, its design quite amateurish and childlike, but I suspect this may be temporary, because click on the ‘PRODUCTS’ tab and the ‘LENTIS | FEMTIS' drop down link currently connects to the Oculentis site.
As I said, I'm not an expert in corporate structure, but I wonder if Oculentis plan to go into administration, or take other steps to swerve responsibility for further expected legal claims?
AND - for whatever reason - should the company no longer exist, where would that leave damaged UK patients needing to access the Oculentis 'Patient Pathway’?
Answer: NHS!
And whilst his recent photo suggests that Oculentis |Teleon MD Mark Lansu has the same (lack of) faith in their lenses as does TopconGB MD Andrew Yorke, the latter has not responding to my perfectly polte and reasonable email last week...
'From: SashaMBEF <sasha@mybeautifuleyes.co.uk>
Subject: Oculentis Patient Pathway (OPP)
Date: 19 August 2020 at 16:27:10 BST
To: Andrew.Yorke@topcon.co.uk
Cc: mark.lansu@oculentis.com, ben.Wanders@oculentis.com
Hi Andrew
Whilst you and your colleagues have refused to respond to my questions since 2015 (selection below), I am aware that both Topcon and Oculentis regularly monitor my social media sites, and have read my recent posts.
Regardless of your hostility toward me, it is a fact that, as patient advocate with My Beautiful Eyes Foundation, I am the only person in the UK providing help and advice to the many thousands of people damaged by refractive eye surgery, thanks to the government’s refusal to address this massive scandal, with corruption at every level (globally).
Within the last week MBEF has been contacted by three more people who recently attended Optical Express stores complaining of visual problems, all shocked to be told that they have the faulty Lentis IOLs - and I have no doubt there are more yet to come!
However, none of these people want to have the ‘free’ treatment offered by OE (paid by Oculentis) and have asked for my advice.
It is possible that litigation could be avoided in at least one of these cases, the person only concerned with having the problematic calcified lenses removed asap, by an experienced surgeon of their choice.
Unfortunately, if they are unable to access the OPP directly, there will be no alternative but to litigate.
With that in mind, I am asking you the same question that Ben Wanders has refused to answer.
Can you please provide me with details of the Oculentis 'Patient Pathway’, and how my clients can access it if not currently in litigation?
I look forward to your earliest response.
Best wishes ?
Sasha Rodoy | My Beautiful Eyes Foundation
Patient Advocate & Campaign Manager'
Last Edit:19 Sep 2021 18:57
by admin
Please Log in or Create an account to join the conversation.
- admin
-
 Offline
Topic Author
Offline
Topic Author
- Posts: 1164
- Thanks: 153
(2 of 3)
Oculentis first notified ophthalmologists of their product recall in December 2014
Halfway down the second page, addressed to surgeons, this sentence screams utter and sickening contempt for patients...
'We are aware that this recall is of inconvenience for you and we would like to thank you in advance for your cooperation'
No apology to patients for their ‘inconvenience’!
And worse, I had sight of another document advising surgeons not to tell patients fitted with the recalled lenses that they were faulty if they hadn't reported problems.
Hence why someone who contacted me a few days ago, fitted with IOLs in 2013, was shocked when he went to Optical Express very recently, complaining of increasing deterioration in his vision since last year, for the optom to tell him he had the #Lentis faulty lenses that have to be removed.
And I have no doubt that there are many more people suffering with deteriorating vision who have no idea it's due to faulty #Oculentis lenses!
Call me old fashioned, but I believe that Oculentis, or Topcon GB, or Optical Express (or other provider), had a moral - and legal - obligation to inform EVERY SINGLE PERSON fitted with the faulty lenses of this fact in 2014, who should then have been offered the opportunity (and cost of) to have the lenses explanted BEFORE they calcified!
Approximately two years ago (time flies!) Oculentis agreed to pay for one of my MBEF clients to have the faulty lenses explanted by a highly experienced surgeon of her choice. However, when they discovered that she intended to litigate, they refused to pay.
They also capped the amount they were willing to pay for an explant, whilst some people have had to turn to the NHS for these ops!
'Those affected typically experience a deterioration of visual acuity at around 36 months post-surgery, calcification of the IOL surface may be observed.’*
'Around 800 people are thought to be affected. The manufacturer claims the problem may be due to an interaction between phosphate crystals used in the hydration process and silicone residues on the lens.’**
'Oculentis says there is evidence some people may be predisposed to this problem or that certain medications can be a factor.’***
www.college-optometrists.org/the-college...lar-lens-recall.html
*Yet many people are presenting with problems more than 84 months (7 yrs) post surgery.
**This means there is the likelihood of hundreds more unsuspecting people still at risk of the lenses calcifying.
***If this were the case, then the MHRA (Medicines & Healthcare products Regulatory Agency) are equally culpable and should be sued, because they are responsible for regulating all medicines and medical devices in the UK by ensuring they work and are acceptably safe.
But this was a bullsh*t excuse from Oculentis - the lenses are faulty, by their own admission!
(European Medicines Agency responsible for Europe-wide licence under the European Commission laws.)
Part 3 of this thread will explain steps taken by Oculentis that I strongly suspect are to divorce the company from all the online bad press, and possibly avoid further claims against them.
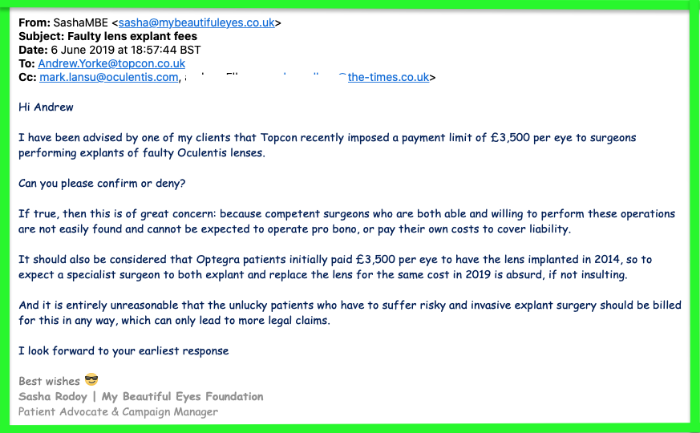
NB: Oculentis distributors TopconGB are responsible for administering the Oculentis Patient Pathway (OPP), yet MD Andrew Yorke refused to answer my questions in 2019 - familiar pattern for these guilty companies!
You will also notice that Andrew Yorke and pals in this LinkedIn pic obviously have no trust in the products they’re pushing!
Oculentis first notified ophthalmologists of their product recall in December 2014
Halfway down the second page, addressed to surgeons, this sentence screams utter and sickening contempt for patients...
'We are aware that this recall is of inconvenience for you and we would like to thank you in advance for your cooperation'
No apology to patients for their ‘inconvenience’!
And worse, I had sight of another document advising surgeons not to tell patients fitted with the recalled lenses that they were faulty if they hadn't reported problems.
Hence why someone who contacted me a few days ago, fitted with IOLs in 2013, was shocked when he went to Optical Express very recently, complaining of increasing deterioration in his vision since last year, for the optom to tell him he had the #Lentis faulty lenses that have to be removed.
And I have no doubt that there are many more people suffering with deteriorating vision who have no idea it's due to faulty #Oculentis lenses!
Call me old fashioned, but I believe that Oculentis, or Topcon GB, or Optical Express (or other provider), had a moral - and legal - obligation to inform EVERY SINGLE PERSON fitted with the faulty lenses of this fact in 2014, who should then have been offered the opportunity (and cost of) to have the lenses explanted BEFORE they calcified!
Approximately two years ago (time flies!) Oculentis agreed to pay for one of my MBEF clients to have the faulty lenses explanted by a highly experienced surgeon of her choice. However, when they discovered that she intended to litigate, they refused to pay.
They also capped the amount they were willing to pay for an explant, whilst some people have had to turn to the NHS for these ops!
'Those affected typically experience a deterioration of visual acuity at around 36 months post-surgery, calcification of the IOL surface may be observed.’*
'Around 800 people are thought to be affected. The manufacturer claims the problem may be due to an interaction between phosphate crystals used in the hydration process and silicone residues on the lens.’**
'Oculentis says there is evidence some people may be predisposed to this problem or that certain medications can be a factor.’***
www.college-optometrists.org/the-college...lar-lens-recall.html
*Yet many people are presenting with problems more than 84 months (7 yrs) post surgery.
**This means there is the likelihood of hundreds more unsuspecting people still at risk of the lenses calcifying.
***If this were the case, then the MHRA (Medicines & Healthcare products Regulatory Agency) are equally culpable and should be sued, because they are responsible for regulating all medicines and medical devices in the UK by ensuring they work and are acceptably safe.
But this was a bullsh*t excuse from Oculentis - the lenses are faulty, by their own admission!
(European Medicines Agency responsible for Europe-wide licence under the European Commission laws.)
Part 3 of this thread will explain steps taken by Oculentis that I strongly suspect are to divorce the company from all the online bad press, and possibly avoid further claims against them.
NB: Oculentis distributors TopconGB are responsible for administering the Oculentis Patient Pathway (OPP), yet MD Andrew Yorke refused to answer my questions in 2019 - familiar pattern for these guilty companies!
You will also notice that Andrew Yorke and pals in this LinkedIn pic obviously have no trust in the products they’re pushing!
Last Edit:21 Jun 2023 14:23
by admin
Please Log in or Create an account to join the conversation.
- admin
-
 Offline
Topic Author
Offline
Topic Author
- Posts: 1164
- Thanks: 153
(1 of 3)
All those responsible for causing damage to many thousands of people with unregulated and risky refractive eye surgery deserve to be prosecuted as criminals
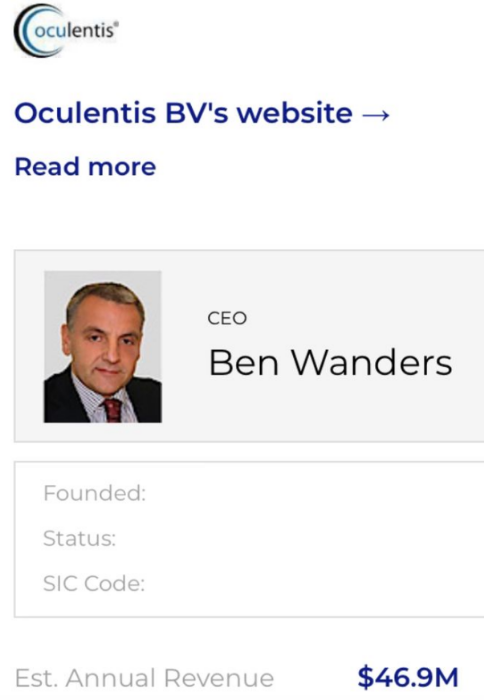
And I include those responsible for the manufacture and sale of faulty intraocular lenses, in this case Dutchman Ben Wanders, CEO and founder of #Oculentis BV, his greed putting profit above the welfare of unlucky #Lentis lens recipients.
‘The Oculentis GmbH/BV is the manufacturer of the famous* LENTIS intraocular lenses with their own research and development department and one of the most modern production facilities for IOL worldwide. The medical technology company has its administration headquarters in Berlin, Germany, whereas it operates its manufacturing, research and development in Eerbeek, Netherlands.’
eyewire.news/articles/20130828-oculentis...business_award_2013/
*Certainly infamous - worldwide!
eyewire.news/articles/oculentis-responds...-lentis-mplus-x-iol/
www.mivision.com.au/2017/10/oculentis-re...n-2012-and-may-2015/
www.thelocal.se/20181127/over-400-swedes...ty-artificial-lenses
'From: SashaOERML <sasha@opticalexpressruinedmylife.co.uk>
Subject: Oculentis Patient Pathway
Date: 22 June 2020 at 16:10:57 BST
To: Ben.Wanders@oculentis.com
Cc: sashaMBEF <sasha@mybeautifuleyes.co.uk>, mark.lansu@oculentis.com
Hi Ben
I am aware that Oculentis recently made a global offer of €1.75m to 130 people fitted with lenses listed on your recall list.
Since posting details on my sites a few days ago I have been contacted by a number of people asking for advice.
This morning I spoke to someone who underwent bilateral lens replacement surgery in 2014 but presented with problems only a few months ago, now advised that the lenses are on the Oculentis recall list and both need explanting.
This contradicts reports claiming that deterioration of visual acuity will typically manifest within 36 months. Are you prepared to comment on this?
Meanwhile, can you please provide me with details of the Oculentis 'Patient Pathway’, and how my clients can access it if not currently in litigation?
I look forward to your earliest response.
NB: Sent from OERML in case MBEF mail not reaching you as I received no response to my previous email below.
Best wishes
Sasha Rodoy | My Beautiful Eyes Foundation
Patient Advocate & Campaign Manager'
No reply!
Then we have the two law firms, who signed up a total of 130 people fitted with the faulty lens, before joining forces and bundling all their clients into the equivalent of a class action claim, even though the majority had NOT been informed that their claim would be dealt with this way.
And when they complained, these twice unlucky people discovered that they had no choice but to accept the arrangement, having signed an agreement before they knew what was intended - huge profit for the two law firms at the expense of the clients!
Said law firms told clients they should jump at the insulting offer, claiming that #Oculentis might be forced out of business if they fought for more.
Somewhat odd therefore, that a number of Oculentis MPlusX claims are being fought by one of these two law firms on their own merits, with settlements way more than the estimated £5-6,000 each of the 130 claimants will end up with.
'The LENTIS MplusX is considered to be one of the better balanced IOL’s available in the market and is used by many renowned and respectable surgeons. It is by no means a “faulty” model of lens as alleged.'
www.topcon-medical.co.uk/files/Local_TGB...tement_Corrected.pdf
Perhaps Ben Wanders and Managing Director Mark Lansu could take a cut in their personal profits for a year, because what they’ve offered damaged patients is nothing less than an insult, showing total contempt for customers (as have the lawyers)!
And before signing away ALL their rights, a question claimants still with recalled #Lentis lenses in their eye/s should be asking: if given access to the Oculentis 'Patient Pathway’ (OPP), will they have a choice of surgeon, or be forced to use someone chosen by Oculentis?**
I strongly suspect the two greedy ambulance chasing law firms won't have bothered asking this question, because once they have their huge profit from this ‘class action’, they won’t give a sh*t what happens to their clients in the future.
When the agreement is signed with Oculentis I intend to publish details of how the approximate €1.75 million will be shared between claimants and law firms: the latter to be named and shamed, unarguably as greedy and unscrupulous as Optical Express et al!
And for their unhappy clients reading here (those who haven’t yet contacted me) details of how to complain to the Legal Ombudsman will be made available - because your (compulsory) agreement to confidentiality does NOT prevent you from complaining about the unsatisfactory service you received from the lawyers!
**To be discussed in part 2.
All those responsible for causing damage to many thousands of people with unregulated and risky refractive eye surgery deserve to be prosecuted as criminals
And I include those responsible for the manufacture and sale of faulty intraocular lenses, in this case Dutchman Ben Wanders, CEO and founder of #Oculentis BV, his greed putting profit above the welfare of unlucky #Lentis lens recipients.
‘The Oculentis GmbH/BV is the manufacturer of the famous* LENTIS intraocular lenses with their own research and development department and one of the most modern production facilities for IOL worldwide. The medical technology company has its administration headquarters in Berlin, Germany, whereas it operates its manufacturing, research and development in Eerbeek, Netherlands.’
eyewire.news/articles/20130828-oculentis...business_award_2013/
*Certainly infamous - worldwide!
eyewire.news/articles/oculentis-responds...-lentis-mplus-x-iol/
www.mivision.com.au/2017/10/oculentis-re...n-2012-and-may-2015/
www.thelocal.se/20181127/over-400-swedes...ty-artificial-lenses
'From: SashaOERML <sasha@opticalexpressruinedmylife.co.uk>
Subject: Oculentis Patient Pathway
Date: 22 June 2020 at 16:10:57 BST
To: Ben.Wanders@oculentis.com
Cc: sashaMBEF <sasha@mybeautifuleyes.co.uk>, mark.lansu@oculentis.com
Hi Ben
I am aware that Oculentis recently made a global offer of €1.75m to 130 people fitted with lenses listed on your recall list.
Since posting details on my sites a few days ago I have been contacted by a number of people asking for advice.
This morning I spoke to someone who underwent bilateral lens replacement surgery in 2014 but presented with problems only a few months ago, now advised that the lenses are on the Oculentis recall list and both need explanting.
This contradicts reports claiming that deterioration of visual acuity will typically manifest within 36 months. Are you prepared to comment on this?
Meanwhile, can you please provide me with details of the Oculentis 'Patient Pathway’, and how my clients can access it if not currently in litigation?
I look forward to your earliest response.
NB: Sent from OERML in case MBEF mail not reaching you as I received no response to my previous email below.
Best wishes
Sasha Rodoy | My Beautiful Eyes Foundation
Patient Advocate & Campaign Manager'
No reply!
Then we have the two law firms, who signed up a total of 130 people fitted with the faulty lens, before joining forces and bundling all their clients into the equivalent of a class action claim, even though the majority had NOT been informed that their claim would be dealt with this way.
And when they complained, these twice unlucky people discovered that they had no choice but to accept the arrangement, having signed an agreement before they knew what was intended - huge profit for the two law firms at the expense of the clients!
Said law firms told clients they should jump at the insulting offer, claiming that #Oculentis might be forced out of business if they fought for more.
Somewhat odd therefore, that a number of Oculentis MPlusX claims are being fought by one of these two law firms on their own merits, with settlements way more than the estimated £5-6,000 each of the 130 claimants will end up with.
'The LENTIS MplusX is considered to be one of the better balanced IOL’s available in the market and is used by many renowned and respectable surgeons. It is by no means a “faulty” model of lens as alleged.'
www.topcon-medical.co.uk/files/Local_TGB...tement_Corrected.pdf
Perhaps Ben Wanders and Managing Director Mark Lansu could take a cut in their personal profits for a year, because what they’ve offered damaged patients is nothing less than an insult, showing total contempt for customers (as have the lawyers)!
And before signing away ALL their rights, a question claimants still with recalled #Lentis lenses in their eye/s should be asking: if given access to the Oculentis 'Patient Pathway’ (OPP), will they have a choice of surgeon, or be forced to use someone chosen by Oculentis?**
I strongly suspect the two greedy ambulance chasing law firms won't have bothered asking this question, because once they have their huge profit from this ‘class action’, they won’t give a sh*t what happens to their clients in the future.
When the agreement is signed with Oculentis I intend to publish details of how the approximate €1.75 million will be shared between claimants and law firms: the latter to be named and shamed, unarguably as greedy and unscrupulous as Optical Express et al!
And for their unhappy clients reading here (those who haven’t yet contacted me) details of how to complain to the Legal Ombudsman will be made available - because your (compulsory) agreement to confidentiality does NOT prevent you from complaining about the unsatisfactory service you received from the lawyers!
**To be discussed in part 2.
Last Edit:27 Aug 2020 14:28
by admin
Please Log in or Create an account to join the conversation.
Moderators: admin, Sasha