REFRACTIVE LENS EXCHANGE aka RLE/NLR +IOLs
- Incyb
- Thank you received: 0
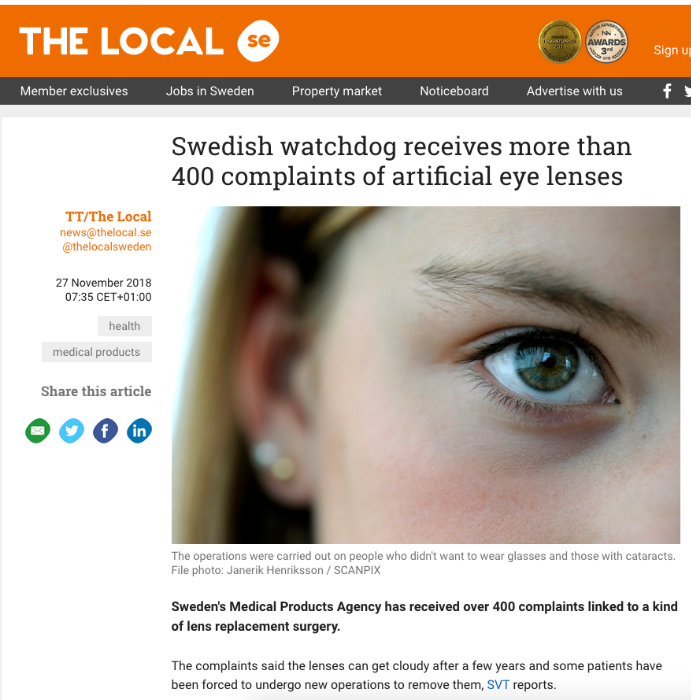
www.thelocal.se/20181127/over-400-swedes...ty-artificial-lenses
Please Log in or Create an account to join the conversation.
- Incyb
- Thank you received: 0
Replied by Incyb on topic Halos, Glare, etc. You know, the fun stuff ...
Posted 02 Dec 2018 15:48 #62Here is a link to an article from 2017, with direct quotes. (Highlights and bold mine.)
"Another important aspect of MIOL (multifocal intraocular lens) or EDOF(extended depth of focus e.g Tecnif Symfony ZXR00) IOL implantation is the perception of postoperative photic phenomena that can potentially compromise patient satisfaction. Generally, when light enters the pseudophakic eye, several mechanisms can disturb the ideal light distribution on the retina. This ultimately leads to a perception of a flawed or imperfect picture or even ghost images and shadows.
Positive dysphotopsia symptoms are described as bright flashes, arcs, halos, or sprinkles of light and are usually strongest in scotopic (dim) light conditions under which the iris dilates
Thus, halos, from a physics standpoint, are INEVITABLE with MIOL and EDOF lenses. This fact is highly relevant, as in a survey Mamalis et al showed that halos and aberrations are a potentially adverse effect whose occurrence represents the main reason for MIOL explantation.
CONCLUSION
It is important that the surgeon asks patients about their work and recreational activities to get as complete a picture as possible of each patient’s predominant visual needs. If, for example, manual or computer work is an integral part of the patient’s lifestyle, good intermediate vision should be supported by the chosen MIOL. If spectacle-free reading ability is strongly desired, an MIOL with a corresponding near addition might be the lens of choice. If both distances should be supported, a trifocal IOL would be suitable. Multifocal IOL defocus curves and capacities (Fig. 2) provide the surgeon and the patient with helpful information for this decision process.
As multifocality is usually linked with increased perception of halo and glare, the patient’s tolerance toward such photopsia also plays an important role. During preoperative consultation, the patient must be informed about the possibility of halo and glare
and the expected intensity for each specific lens type. A more relaxed personality with a readiness to compromise often indicates
higher compatibility with MIOLs, especially trifocal lenses, than a hypercritical and obsessive patient"
Interesting article which makes me wonder -
Were YOU asked in detail BY THE SURGEON (or indeed the office tea-lady) about your work and recreational activities with a view to establishing whether you could tolerate these side-effects and their impact?
Were you told halos were "inevitable", as they are according to the quote above from this scientific article?
I wasn't.
Please Log in or Create an account to join the conversation.
- Incyb
- Thank you received: 0
Replied by Incyb on topic Lens Replacement Surgery and the "REAL" risk of P. C. O and Yag
Posted 02 Dec 2018 14:56 #63The above article states that Posterior Capsular Opacification (which causes vision to become cloudy) is a COMMON long-term complication of cataract surgery (which includes IOLs in this article)
"The rates of PCO following surgery are estimated to be 12% at 1 year, 21" a year 3 and 28% at 5 years after surgery".
I don't know about you, but 28% is an unacceptably high risk to me. It is glossed over in the "OE consent" documentation. How many people would say "No Thanks" if they were told the statistics?
The article goes on to say that treatment with YAG laser capsulotomy is effective, HOWEVER, complications, including retinal detachment, macular oedema, and increases in intraocular pressure, may also occur. The risk of retinal detachment is well known to be higher in myoptics i.e short sighted people.
If a retina detaches and cannot be repaired, you go blind in the affected eye.
Please Log in or Create an account to join the conversation.
- Fuller
-
Offline
- Posts: 1
- Thank you received: 0
My eyes are watery, they feel sore and prickly. I feel like they are constantly being strained. In the morning my right eye is stuck together. My sight for reading is becoming increasingly difficult (this was the only part I was originally happy about)
When I read my eyes become painful, blurry and I can see something I can only describe as ‘blobs’.
My distance was never good after the op, but I feel it is deteriorating. I can only work on the computer for short periods of time. I intended to work full time after this op to help pay back the loan. I haven’t been able to do this, as my work consists of reading and modifying for profoundly deaf students and I know the pain would be too much.
Optical Express use hard selling tactics and then cover themselves in the small print.
My eyes are ruined, I’m in constant pain. I’m in debt. I paid a lot of money for a product that has not worked and put me in a worse situation than before the op financially and physically.
I am left with very painful eyes, SPK, clouding behind one eye and still wearing glasses. Nightmare!!!
This is just a brief summary of my experience. I thought 7 pages may be too long!
______________________
admin: For help/advice, email sasha@opticalexpressruinedmylife.co.uk
Please Log in or Create an account to join the conversation.
- AJ999
-
Offline
- Posts: 1
- Thank you received: 0
I thought i would use this forum to give you my horror story with Optical Express (OE) so that others going through this awful experience know that they are not alone.
I work for the emergency services and decided to go to the free consultation with OE as they seemed like a reputable company that carried out a lot of eye surgeries successfully. When i arrived at the consultation with the idea of getting laser eye surgery to reduce my dependancy on glasses for my job, i was quickly informed that my eyesight was far too poor for that surgery however, i could get RLE surgery. When i was told it would cost me in excess of £6,500 i laughed and walked out as my public servant wage did not deliver those monies.
Within days i was offered a lower price with a better payment plan. I see the sales tactic now but at the time i was blind to it - no pun intended. My wonderful elderly father gave me the cash as an early inheritance and i booked the surgery, as i trusted what had been told to me. I was told of the minimal chances of any or all complications being less than 1% and told in the majority of cases these complications diminished as the patient healed. This included the minimal risks of haloes/glares/starbursts.
Within a day post op i knew something was not right. My vision was poor near and distance, i had day time glares and severe night time glares/haloes and starbursts, so much so that i could not return to work as the alleged 3 day recovery quoted suggested. In fact it would be three months before i returned to work. During this period I was frequently told that these symptoms would dissipate as time passed as it was very rare for them to remain. It was all down to my brain neuroadapting. Now this is a word that i had never heard of pre-op. After 9 months of having poor day time sight and having to wear glasses plus awful night time vision and a significant drop in confidence when driving at night, i was finally told i would need the multifocal lenses taken out and replaced with monofocal lenses. The risks were high. Higher than the previous surgery but i was advised it wasn't an uncommon procedure carried out there.
Obviously by this time i had lost all trust in OE and thankfully had found this site, so i started to research more into this phenomenon and found out the chances of haloes/glares/starbursts was nearly 40% in some studies!! In fact one of the OE surgeons stated he usually tells all his patients that they will experience these symptoms after this type of surgery as it is so common!!
If i had known about this i would NEVER of had the surgery as i am facing the prospect of losing my job if i don't have the risky explantation surgery. People may say, well its obvious why you say that now, hindsight is 20:20 (sorry). However, prior to the surgery i discussed monofocal implants instead of multifocal implants with the OE staff, as it was a cheaper option but decided against it as i was told my brain had to adjust to the lenses being at different focal lengths (which made sense) and this could take an unspecified length of time. This shows that i would have decided against any surgery that had a significant risk to getting back to my work quickly. And i was repeatedly told the risk of any complication was 1% and a percentage of that 1% had a risk of glares/haloes with an even smaller percentage of them not recovering. So going by that the risk of having glares/haloes permanently was less than 0.5%. Plain faced liars.
Currently i am weighing up my choices of whether to have the risky explantation surgery (10-20% chance of blindness) within the NHS or keep the eyesight that i have been left with and lose my job that i have loved for 20 years. Not an easy choice to make......
Please Log in or Create an account to join the conversation.
- Audrey
Hi,Guy From North Wales wrote: Can I say thank you to those of you that have replied, sometimes you can read stuff and think how can they get away with all this. We live in a country where so much stuff is questioned under wether its politically correct and yet here is a company getting people to sign with no idea what they are signing because they are so carried away with this idea of better vision, the quality of the establishments that yes most of us get dragged in. But this isnt our nails or bigger breasts, its not cosmetic this is one of the most important things our vision. So for me some replies have really really helped and I can see I am not alone and indeed reading through the forum can actually see I am probably quite lucky.
Claire Foley I am going to try the doctors, mainly because I have psoriasis which is also stress related and this isnt in anyones interest the fact I am worried about my vision and losing one eye sight and not being able to drive. I went private rather then asking the NHS so now it has gone wrong I am not going to be proud but ask for help.
I'm in a similar position to yourself in that I had rle surgery and suffer from.bad glares/haloes which mean I need explantation surgery through the nhs. However the risk of retinal detachment [20%] terrifies me. Is this the kind of figures you received?
Please Log in or Create an account to join the conversation.
- admin
-
 Offline
Offline
- Posts: 1163
- Thank you received: 153
On 7 March I wrote, 'Bernie Chang, employed by Optegra, most definitely had an inextricable COI, and in my opinion therefore could not be considered ‘independent’, so why was he allowed to be part of the investigation?'
Not only was Bernie Chang previously Vice President and Chair of the Professional Standards Committee at the RCOphth, and one of the core members of the RCOphth Refractive Surgery Standards Working Group (RSSWG), he also colluded with David Moulsdale, and others, to remove me from my nominated position as RSSWG lay adviser in 2015*.
Not forgetting that OE’s very own Medical Director David Teenan** was also a core member of the RSSWG, 'to keep OE on board' otherwise they wouldn’t agree to the new standards, as Vice Chair Peter Tiffin told Shadow Chancellor John McDonnell during our meeting at the College on 4 December 2015 (arguing for my reinstatement after removal without right of reply).***
Points of concern:
• MHRA had discussions with and took advice from the RCOphth about the safety of the Oculentis MPlus X lens sold by OE and Optegra.
• Both companies have numerous legal claims from Px fitted with the multifocal MPlus X lens.
• Bernie Chang closely allied with Optegra and RCOphth.
• RCOphth has an indisputable track record proving that their allegiance is with members, not for patients damaged by their members.
• People still continue to contact me in 2018 reporting problems with the MPlus X lens (and other MF lens too).****
• MHRA is largely funded by drug and device manufacturers, the UK equivalent of the FDA - and equally as corrupt in my opinion.
'MHRA is funded by the Department of Health and Social Care for the regulation of medical devices, whilst the costs of medicines regulation is met through fees from the pharmaceutical industry. This has led to suggestions by some MPs that MHRA is too reliant on industry, and so not fully independent.’
en.wikipedia.org/wiki/Medicines_and_Heal...ts_Regulatory_Agency
Taken from a Daily Mail article published in February, 'The MHRA, along with other healthcare regulators around Europe, relies on a network of 59 commercially run ‘notifying bodies’ to approve the safety and efficacy of medical devices. But as Good Health has reported, the notifying bodies are paid by manufacturers to win approval for their products and the requirements for scientific validation are vague, say experts.
When asked, an MHRA spokesperson said: “We protect and improve the health of millions of people every day through the effective regulation of medicines and medical devices, underpinned by science and research.
Our role is not to protect industry interests. We have a full and transparent conflicts of interest policy which means that our staff cannot hold any interests in the pharmaceutical industry during their employment with MHRA."
MHRA chief executive Dr Ian Hudson told Good Health: “It is important to review and learn from how the healthcare system and regulators have handled these three issues, including how we ensure the patient voice is carefully heard. We will work closely with DHSC, the NHS and the wider healthcare system on this review. By all parts of the healthcare system working together, we can ensure patients are listened to and their concerns are addressed.”'
www.dailymail.co.uk/health/article-54384...s.html#ixzz5D36XmE6s
I repeat, the stink of corruption oozes from every level of this sickening and unregulated industry, globally, and - quite frighteningly - extends to the government and EVERY single health organisation that the public are lead to believe they can trust!
* ‘others' notably included Moorfields surgeon and RSWWG Chair Bruce Allen, as subsequent SARs to Moulsdale and the RCOphth disclosed. And quite surprisingly to me at the time, David Moulsdale's disclosure was more honest than that of the College, whose CEO Kathy Evans dishonestly edited emails to keep info from me that I was entitled to.
**Top of the leader board with numerous RLE legal claims against him in Scotland.
*** OE didn't agree, don’t follow them, and have written their own standards with FODO.
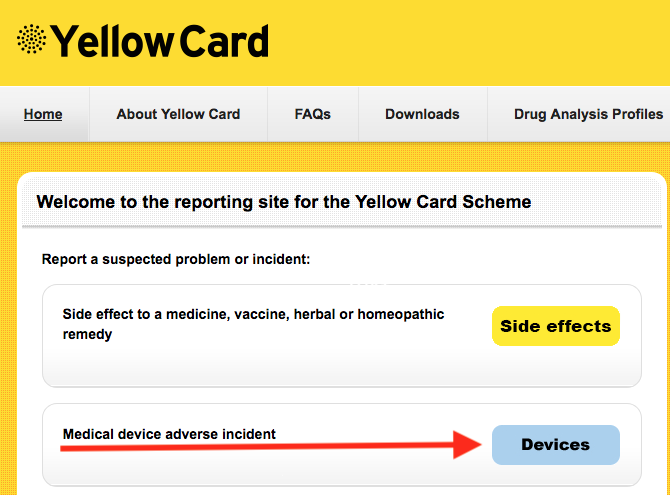
**** Please contact me if you have any issues with implanted lenses. NB: VERY important that you report this to the MHRA via the Yellow Card Scheme @ yellowcard.mhra.gov.uk
Meanwhile, you may have noticed that my posts are fewer than usual. This is due to severe eye pain I've recently been suffering, so bad that I have to ration my time spent at the computer. And now I have hay fever to make it even worse!
Very frustrating because I have a backlog of news to post - and to those now breathing a huge sigh of relief because they KNOW they’re on my list, enjoy the reprieve while you can, it’s temporary!
Please Log in or Create an account to join the conversation.
- admin
-
 Offline
Offline
- Posts: 1163
- Thank you received: 153
Without cataracts this surgical procedure is termed refractive lens exchange (RLE), or natural lens replacement (NLR).
And as mentioned at the end of my 7 March post, it's not just the infamous Oculentis Mplus X lens that are problematic.
• December 2014, multinational pharmaceutical corporation Ocuentis issued an urgent Field Service Notice (FSN) and recall of its LENTIS HydroSmart intraocular lens.
• August 2016, multinational health care company AMO* (Abbott Medical Optics) issued a voluntary recall of intraocular lenses, following the detection of an inspection equipment malfunction that resulted in a total of 737 globally distributed lenses not being fully checked prior to release. FSN2016-02: Model Numbers ZCB00, PCB00, AR40e, ZLB00, ZXR00.
• September 2017, Oculentis issued a further Field Safety Notice applying to all LENTIS IOLs with model numbers starting with L-, LU- and LS- and expiry date between January 2017 and May 2020. Allegedly the lenses which have suffered opacification due to calcification are defective due to contamination in the manufacturing process.
As a patient advocate, over recent years I have spoken with countless numbers of people who have had - or need to have - their multifocal lens(es) explanted, not only those listed above.
A bilateral explant can cost £11,000 privately, beyond what the majority of damaged patients can afford to pay, so more frequently this is happening at significant cost to the NHS.
If you have post op complications with IOLs, and need further tretament, speak to a specialist lawyer before agreeing to anything with the provider, because you may be entitled to compensation, especially if the lens is proven defective.
In addition, it is important that you report the issue to the MHRA via the Yellow Card Scheme @ yellowcard.mhra.gov.uk
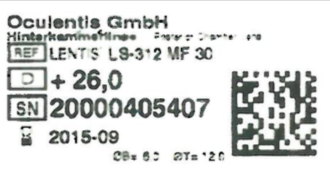
NB: To do this you need to know which IOL you have, and should have been given a lens ID sticker at the time of surgery. If not, you are entitled to ask the provider for this at no cost.
Optegra market refractive lens exchange under the Clarivu brand name, claiming, 'This proven treatment, carried out thousands of times by Optegra, is a technically advanced and sophisticated development of cataract surgery, which is one of the world’s most frequently performed surgical procedures.'
www.optegra.com/treatments/lens-replacement-surgery/clarivu/
Notice the absence of the 'safe’ adjective: one truth amidst their hype, because increasing numbers of Optegra damaged patients have contacted me in recent months, who are, in my opinion, being treated no better than OE's damaged patients.
Clarivu is unconscionably promoted by Ruth Langsford, lucratively paid over the years to tell people - without caveat - 'Do it, do it, do it!'.
And regular readers will remember that I met Ruth at one of Optegra's promotional dinners in 2015, her nauseating sales pitch intended to persuade people to sign up for Clarivu surgery by the time dessert was served - and yes, she actually said, 'Do it, do it, do it!'.
Before I was 'asked to leave', I challenged Ruth, asking how she'd feel if a person who'd had surgery because of her promotion was left with damaged eyes.
She insensitively replied that it would be nothing to do with her, saying, 'It’s like promoting shampoo, it might work for me but not for them'. (recorded)
Yet no such caveat in her advertising!
www.opticalexpressruinedmylife.co.uk/ind...a-clarivu.html#13850
And this conveniently leads me back to the MHRA, which I'll discuss further in due course.
*In 2016 Johnson & Johnson acquired AMO/Abbott Medical Optics for $4,325 billion!
www.jnj.com/media-center/press-releases/...bbott-medical-optics
Hence the ongoing fight to expose this corrupt and dangerous industry, because Big Pharma have plenty of cash to bribe government and health care organisations we naively believed were there to protect us.
No conspiracy theory - scarily the truth
For advice contact:
info@mybeautifuleyes.co.uk
info@opticalexpressruinedmylife.co.uk
Please Log in or Create an account to join the conversation.
- admin
-
 Offline
Offline
- Posts: 1163
- Thank you received: 153
February 2015, Optical Express issued legal proceedings against Associated Newspapers Ltd over alleged libel in the Daily Mail article published on 5 January 2015, headed, ‘Blindness fears over eye surgery at High St clinic’.
The original and lengthy article about the problematic Oculentis Mplus X lens, written by journalist Daniel Boffey, was published in the Sunday Observer hard copy and Guardian online on 4 January 2015. (read history)
Sadly* OE suddenly dropped their £21.5 million claim in February 2017, and accepted a Part 36 offer of £125,000 that had been on the table since 2016.
*OERML lost guaranteed national media/press cover - though there was an upside when OE argued costs and the judge ruled in the defendant’s favour, costing OE approx £1 million!
www.civillitigationbrief.com/2017/11/05/...ages-from-the-outset
www.clydeco.com/blog/insurance-hub/artic...ssociated-newspapers
On 5 January Other newspapers published a mini version of Daniel Boffey’s exposé, including the Daily Mirror.
And you might well ask why Optical Express didn’t sue the Mirror for libel too! I was told by someone in confidence that it was (allegedly) because of the Scottish connections.
And so the furore died down, with the press all too scared to report on anything remotely related to Optical Express...
Then, last year, a journalist contacted the MHRA, intending to write a follow up to this story, but he was told that there was no investigation into the Mplus X lens!
Really?
4 March 2015, I emailed the MHRA with these questions concerning their 'non existent' investigation...
• Q: What is the MHRA’s criteria for independent experts?
MHRA: The MHRA has access to a network of specialist healthcare professionals who are experts in their field. They are subject to confidentiality agreements and have to confirm any potential conflicts of interest. We also engage collaboratively with professional bodies as part of our mutual commitment to patient safety.
• Q: Have you contacted the RCOphth?
MHRA: The MHRA Devices Clinical Team is currently in the process of engaging with the Royal College of Ophthalmologists and other stakeholders.
(I trusted the RCOphth at that time, not yet aware of the corruption within the College)
• Q: Will the experts’ names be made public when the investigation is complete?
MHRA: Due to confidentiality names of external experts contacted as part of our investigation will not be published. This is to ensure they are not contacted by third parties who might seek to influence their opinions.
And from an email I received on 5 March 2015...
'Dear Mrs Rodoy,
As discussed, our investigation of the Mplus X lens is on-going.
Kind regards,
Ian M Smith
Senior Device Specialist
Biosciences & Implants
Devices
Medicines and Healthcare Products Regulatory Agency'
1 April 2015, I had also again asked if the experts’ names would be made public when the investigation is complete - when it is too late to for third parties to influence their opinions.
MHRA: 'Experts contacted as part of this investigation may also be contacted for future investigations. Their names will not be published to ensure that they are not influenced by third parties during future investigations and to ensure that they agree to remain as independent experts to the MHRA. Inability to attract suitably qualified experts to participate in investigations would represent a risk to the agency’s ability to conduct its work and therefore present a risk to public health and safety.'
26 Nov 2015, I wrote, 'I have been advised that ophthalmologist Bernard Chang, employed by Optegra who have themselves sold this lens to many patients, is one of the experts investigating the MPlus X lens complaints.
You told me that you will not publish the names of the experts even after the results of the investigation, but can you at least assure me that all experts have disclosed their COI.'
24 December 2015, the MHRA response I received was vague, 'We can confirm that all external experts consulted by MHRA are asked to inform the agency of any conflicts of interest.'
Bernie Chang most definitely had an inextricable COI, so why was he allowed to be part of the investigation?
11 March 2016, to me from the MHRA...
'Dear Mrs Rodoy,
Thank you for your e-mail of 19 February.
Please see below responses to your questions:
• What is the current status of the MHRA's investigation into the Mplus X Lens manufactured by Oculentis?
• Is the investigation open or closed?
MHRA continue to monitor for any further incidents and signals that may add evidence to the investigation. It is for the surgeon to determine which patients are suitable to receive this type of lens based on the manufacturers’ instructions for use and their own clinical judgement.
• If closed, will a report be published on the MHRA's findings?
Reports are not published on individual investigations. The MHRA’s procedure for adverse incident investigation is to maintain any information in relation to a particular investigation on an adverse incident database record. This information is then subject to on-going trending and surveillance.
• Will such a report be publicly available?
Please see response above.
• How many reports ('yellow card' medical device adverse incident reports or any other reports) has the MHRA received relating to issues with the Mplus X lens?
As the manufacturer of this lens is still in existence this information is subject to the exemptions contained in section 44(1) (a) of the Freedom of Information Act (FOIA). In particular Section 237(2) of the Enterprise Act 2002 applies to specified information, which is defined in section 237(1) and section 238 (by reference to Schedule 14 and the regulations made under section 11 of the Consumer Protection Act 1987) to include information held by MHRA and relating to any business of an undertaking. Section 237(2) provides that such information must not be disclosed while the undertaking continues in existence. There are a number of exceptions to this general rule, in Part 9 of the Enterprise Act 2002. The MHRA’s position is that none of these exceptions apply to the information that you have requested.
As this is an absolute exemption it is not subject to a test of public interest.'
I then asked, if the MHRA is unable to share this information with me perhaps the Shadow Chancellor John McDonnell could request the information?
Nope!
So there's been a secret investigation into a dodgy medical device that has damaged so many people's eyes but the report will also be kept secret?! You really couldn't make it up!
Simply for the purpose of transparency and accuracy, two days ago I wrote to the MHRA...
'Can you please update me on the conclusion of this investigation as I am unable to find details on your website.
I am also concerned that a journalist claims that when he contacted the MHRA last year he was advised that there has never been an investigation into the Mplus X lens, only yellow cards filed.
I look forward to your response.'
I will of course publish the MHRA response, 'within 20 days' as they advised me.
The stink of corruption oozes from every level of this sickening and unregulated industry, globally, and - quite frighteningly - extends to the government and EVERY single health organisation that the public are lead to believe they can trust!
PS: My next post will include an important message for anyone suffering post op problems with lens implants (IOLs), no matter who the provider, or which type of lens, because it's not just the Mplus X people are having issues with!
As Carl G also wrote, if you haven't got cataracts - don't do it!
Please Log in or Create an account to join the conversation.
- Carl G
-
 Offline
Offline
- Posts: 27
- Thank you received: 7
Talk soon.
Please Log in or Create an account to join the conversation.